Monkeypox: White House to target Pride events with extra vaccines in major strategy overhaul
The White House is hoping to get more monkeypox jabs in LGBTQ+ people’s arms during Pride. (ichael M. Santiago/Getty Images)
LGBTQ+ Pride events in the US are to get more monkeypox vaccines as part of a new targeted approach by the White House.
The Biden-Harris administration is hoping to prevent the disease from spilling out from high-risk groups to the broad population by better educating and vaccinating LGBTQ+ people as part of a pilot programme.
State and local health jurisdictions in areas hosting Pride events will receive a vaccine boost as the Department of Health and Human Services unlocks 50,000 doses set aside from the Strategic Nation Stockpile, the White House said Thursday (18 August).
Health experts hope this will curb an outbreak that has so far infected at least 13,000 Americans, according to the Center for Disease Control and Prevention (CDC).
As hundreds of thousands of people prepare for big dates in the Pride calendar, federal officials are working with health departments in North Carolina, Georgia, and Louisiana to send out an increasingly urgent warning about monkeypox.
The trial run will begin Saturday (20 August) August at North Carolina’s Charlotte Pride which attracts tens of thousands each year. North Carolina will be given 2,000 doses for the two-day event alongside the 18,000 already allocated.
At each event, which includes New Orleans’ Southern Decadence and Atlanta Black Pride, the CDC will help organisers set up vaccine pop-ups and promote information about monkeypox to get as many shots in people’s arms as possible.
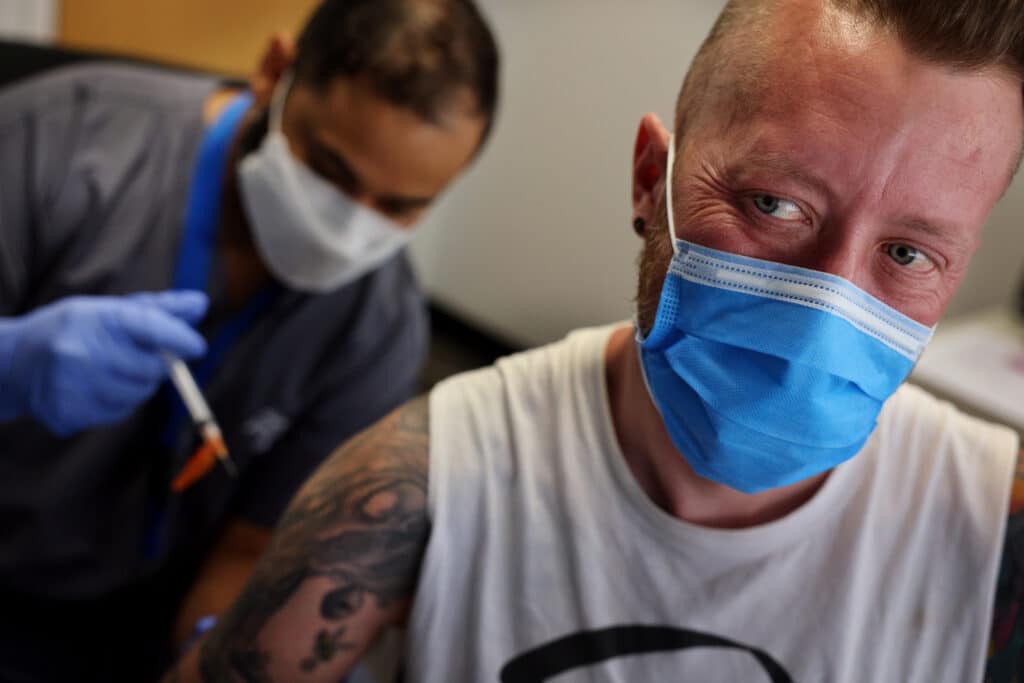
One way the administration wants to do this is by injecting the vaccine a little differently. Injecting a smaller amount of the vaccine between layers of the skin, instead of the underlying flat, could free up hundreds of thousands more doses, health officials estimate.
This came after the federal government issued a public health emergency, enabling the Food and Drug Administration to give the method the green light.
“Since the first case was confirmed in the United States, the Administration has led a whole-of-government response to make tests, vaccines, and treatments more widely available to communities across the country and has worked with the LGBTQI+ community to provide information and resources directly to communities most at risk of contracting the virus,” the White House said.
Dr Demetre Daskalakis, the White House’s deputy director for monkeypox response, told LGBTQ+ Nation that giving queer men the know-how about the disease is also crucial.
“With public health being really local, we’re going to make sure that we provide them what they need in terms of education, outreach materials and any technical assistance to be able to work on the ground to make sure that we’re providing folks with culturally appropriate information about how to prevent monkeypox, and also awareness of the disease,” he said.
“Part of that package is definitely really clear advice around safer sex and safer gatherings. To make sure that it’s extraordinarily clear, given what we know about the data, that this is affecting gay, bisexual and other men who have sex with men, and that a lot of the transmission has been in the context of sex and sexual activity.”
According to the World Health Organization, monkeypox is transmitted from person to person via close contact with lesions, body fluids, respiratory droplets and contaminated materials.
The new outbreak, which flared up in May, has mainly spread between men who have sex with men. Often through the kind of close, intimate contact that only comes with sex, researchers say.
America’s response to monkeypox has been largely undermined by a global vaccine shortage, one that the nation may have been able to bypass altogether.
Less than a decade ago, the US had some 20 million doses of the monkeypox vaccine, according to a 2014 report by biotech company Bavarian Nordic, the world’s sole producer of the monkeypox vaccine Jynneos.
But over the years, the supply dried up as doses expired as officials waited on the FDA to approve a freeze-dried version but didn’t replenish the dwindling reserve.
The federal government has shipped out only 670,000 doses to local health jurisdictions so far, covering only one-third of the 1.7 million people the CDC has identified as high risk.